OPTIMIZE ME CHALLENGE
Optimize Me! Wellness and Body Transformation Challenge Launches on 9/24/24
Thank you for your responses. Next step is to take the survey that best fits your health improvement goals below.
1) I would like to optimize my health. click here: https://bit.ly/3B66mcf
2) I would like to optimize fat loss and transform my body. Click here: https://bit.ly/3Zhm2DU
Oops, there was an error sending your message.
Please try again later.
Please try again later.
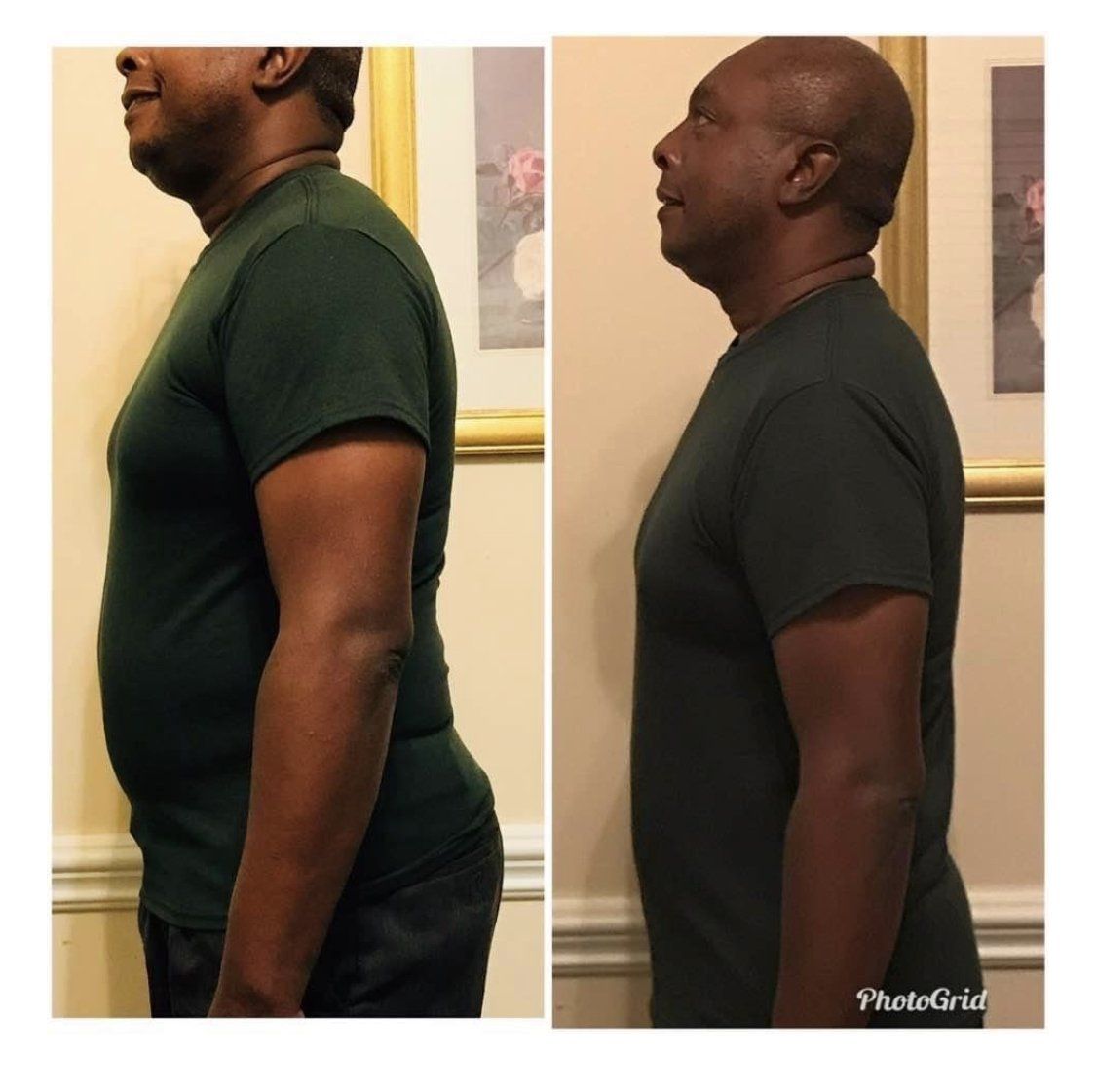
This challenge is about Optimization! Getting Results!
This challenge is about Optimization! Getting Results!
I have been in wellness since May of 1998 and I have never seen so many people so sick or have so many health challenges. With most of the nation overweight or obese, the health of America is in decline. This 12 week challenge is about getting rid of the ok or doing alright mentality with regards to your health. We should be doing great or amazing. Doing great is the new normal when stop putting ourselves last and make our health a priority. You will learn things in this session that will rock you to the core. It will be fun, exciting, and full of surprises. There is not a one size fits all mentality here. If you desire to shed weight, then that is your challenge. If you desire better gut health, then that is your challenge. Some of you make reach your goal in 60 days and desire to work on another goal, that is perfectly fine because you met the challenge. We are going to cover so much over the 12 weeks that I would hang around until the end to celebrate everyones success. I am sure there will be some great testimonies at the end.
We will launch with an overview on September 24th with a limit of 15 people accepted. While I would love to help everyone, I will not accept anyone the does not complete either the weight loss profile or the nutri-physical health analysis
because if you will not take 3-5 minutes to take a survey to help yourself, then it will be hard to optimize your health. This will be a safe space and all that participate will receive support. We are science based, clinically proven, AAA+ BBB rating winning the Torch Award back to back. Needless to say, there are endless testimonies as well. Having worked in the medical field I am HIPPA compliant and do not share client information.
The science behind our advanced nutraceuticals ensures that Health Professionals, their patients , and you, receive the most effective products on the market today.

Slide title
Write your caption hereButton
Slide title
Write your caption hereButton
This is why you can trust us. We take extreme measures to make certain our products are pure and effective.
This is why you can trust us. We take extreme measures to make certain our products are pure and effective.
Creating a superior supplement begins with quality ingredients. The raw materials used in Isotonix products undergo a comprehensive evaluation by our manufacturers to ensure that they meet stringent purity and potency requirements. Verification and approval by a Certificate of Analysis (COA) is required for each raw ingredient. Approved raw materials are subject to laboratory confirmation of physical characteristics and material potency, as well as analysis for microbiological contamination and heavy metals.
Each product is then carefully prepared following a written master manufacturing record, and every batch is reviewed and approved by quality control personnel. Products are inspected upon receipt for damage, label accuracy, appearance, taste and identity prior to release.
Each of our production facilities is required to meet GMP (Good Manufacturing Practice) standards. This means they are periodically inspected and approved by either the Food and Drug Administration or the United States Department of Agriculture for their strict adherence to Standard Operating Procedures, employee training, product specifications, and supplier/vendor qualification procedures.
All manufacturing equipment that comes in contact with our products must be cleaned according to mandated procedures between production runs and verified to ensure sanitation has been performed prior to use. Product samples are also collected at various stages of production for chemical and microbiological analysis to ensure consistency and purity. Lot code confirmation, weight checks, label and material checks are performed according to exact written procedures.*
- Mon - Fri
- -
- Saturday
- Appointment Only
- Sunday
- Closed